L-alanine as food additive
General description
L-alanine is the L-enantiomer of alanine. It has a role as an EC 4.3.1.15 (diaminopropionate ammonia-lyase) inhibitor and a fundamental metabolite. It is a pyruvate family amino acid, a proteinogenic amino acid, a L-alpha-amino acid and an alanine. It is a conjugate base of a L-alaninium. It is a conjugate acid of a L-alaninate. It is an enantiomer of a D-alanine. It is a tautomer of a L-alanine zwitterion. Alanine is a non-essential amino acid that occurs in high levels in its free state in plasma. It is produced from pyruvate by transamination. It is involved in sugar and acid metabolism, increases immunity, and provides energy for muscle tissue, brain, and the central nervous system. L-Alanine is a metabolite found in Escherichia coli (strain K12, MG1655).
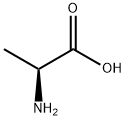
Figure 1 the chemical structure of L-alanine
L-alanine is also called L-alanine- α- Aminopropionic acid is one of more than 20 kinds of amino acids that make up human protein. It is also the amino acid with the highest content in human blood.The molecular formula of L-alanine is CH3CH (NH2) COOH, the molecular weight is 89.09, the density is 1.432 g / cm3, the melting point is 297 ℃, the appearance is colorless to white crystalline powder, soluble in water, slightly soluble in ethanol, insoluble in ether and acetone, odorless and non-toxic, with fresh and sweet taste, in which the sweet taste is 1.2 times that of sucrose.
Application and Pharmacology
L-alanine is a neutral non-essential amino acid. It can not only be used for the protein synthesis, but also can be used as flavor enhancer, preservative and preservation agent in the food field, as pharmaceutical raw materials in the pharmaceutical field, and in the synthesis of mild surfactants in the field of daily chemical.
L-Alanine is both a nitrogen (N) donor by transamination to pyruvate and a N receptor via transamination of pyruvate with a variety of amino acids. Skeletal muscle synthesizes and releases alanine in large amounts, while the liver is the primary site for alanine uptake. 15 Alanine is also produced by the kidney and the gut. 3,4,6 Thus, interorgan transfer is a key feature of alanine metabolism. Presumably, the source of pyruvate for alanine formation originates from glucose via glycolysis. Once alanine has been deaminated to form pyruvate, the pyruvate can be either (1) reduced further to lactate, (2) decarboxylated to form acetyl-coenzyme A, or (3) carboxylated to form oxaloacetate. The oxaloacetate entering the citric acid cycle may be oxidized to CO2 or may provide carbon for gluconeogenesis. The net deamination of alanine leads to formation of urea in the liver, which is also the primary site for gluconeogenesis from the pyruvate carbon that is produced[1].
So far, nine disease-associated triplet extensions have also been described for L-alanine codons. The extensions lead primarily to skeletal malformations. Eight of these proteins represent transcription factors, while the nuclear poly-adenylate binding protein 1, PABPN1, is an RNA binding protein. Additional L-alanines in PABPN1 lead to the disease oculopharyngeal muscular dystrophy (OPMD). The alanine extension affects the N-terminal domain of the protein, which has been shown to lack tertiary contacts. Biochemical analyses of the N-terminal domain revealed an alanine-dependent fibril formation. However, fibril formation of full-length protein did not recapitulate the findings of the N-terminal domain. Fibril formation of intact PABPN1 was independent of the L-alanine segment, and the fibrils displayed biochemical properties that were completely different from those of the N-terminal domain[2].
Synthesis
At present, the main production methods of L-alanine include chemical synthesis, hydrolytic extraction, enzyme transformation and microbial fermentation. The quality of L-alanine produced by chemical synthesis was poor, and the production process was easy to cause environmental pollution. The production process of hydrolytic extraction method was complicated, and it is not suitable for large-scale and industrial production. The enzymatic conversion method required L-aspartic acid as raw material, and the cost was higher. In comparison, microbial fermentation method is more suitable for industrial production.
The research progress of L-alanine production by microbial fermentation. The production of L-alanine by fermentation is mainly studied from the construction of high-yield strains, the optimization of fermentation conditions and the selection of extraction methods. The core problem is the construction of high-yield strains, the selection of appropriate strains for genetic transformation, and the generation of by-products is reduced and the yield of L-alanine is improved by deleting the genes related to the carbon source competition of L-alanine. At present, E. coli is the most widely used strain for genetic transformation. E. coli has the advantages of clear genetic background, easy genetic engineering operation, rapid reproduction, low growth requirements, low cost and safety. It is a good original strain. The advantage of fermentation method is that the carbon source glucose used is a low-cost renewable resource. Some people also began to study the use of mixed sugar in cheap lignocellulose as a carbon source in order to reduce the production cost[3,4].
References
1.Winter R., Liebold J. & Schwarz E., "The unresolved puzzle why alanine extensions cause disease," bchm, Vol.394, No.8(2013), pp.951-963.
2.Battezzati A., Haisch M. & Brillon D. J. et al., "Splanchnic utilization of enteral alanine in humans," Metabolism, clinical and experimental, Vol.48, No.7(1999), pp.915-921.
3.Shanghaitao, Xubin: a method for producing L-alanine, 2013.
4.Nie Yupeng, Xu Hui, Yao Mingjing, etc.: overview of the application and production status of L-alanine, China Brewing, No. 11, 2021, pp. 26-30.
You may like
Related articles And Qustion
Lastest Price from L-Alanine manufacturers

US $0.00/kg2025-06-19
- CAS:
- 56-41-7
- Min. Order:
- 100kg
- Purity:
- 99%min
- Supply Ability:
- 1 tons

US $0.00/Kg/Drum2025-04-21
- CAS:
- 56-41-7
- Min. Order:
- 1KG
- Purity:
- 98.5%-101.5%
- Supply Ability:
- 10 TONS